Antimicrobial resistance (AMR) is worldwide threat. But how does AMR happen?
Bacteria are tenacious. Like every other life form, they fight to survive. Over billions of years they’ve been highly successful, colonising every habitat on Earth, from Arctic ice to hot springs, rock to ocean and, of course, animals, plants and people.
To do that, bacteria have had to evolve defences against natural enemies like those in the penicillin mould that has been widely used in antibiotic therapy since last century. This is antimicrobial resistance (AMR).
But while a human generation is around 30 years, for microbes it’s closer to 30 minutes. They adapt to environmental threats like antibiotics quickly; global geospatial modelling estimated that 40 billion daily antibiotic doses were given to people alone in 2018, representing an enormous threat to the microbes’ survival.
“When bacteria encounter a drug that will kill them, the survivors evolve resistance to the drug,” says Professor Trevor Lithgow from Monash University’s Centre to Impact AMR.
“
When bacteria encounter a drug that will kill them, the survivors evolve resistance to the drug.
Professor Trevor Lithgow
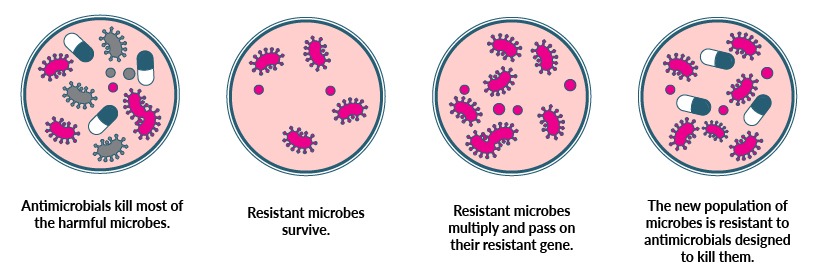
If antimicrobial resistance (AMR) occurs in nature, what makes it such a big problem now?
Antibiotics became a game-changing weapon against infection, saving countless millions of lives. But microbes are hardwired to fight back, against both natural and pharmacy-made threats.
It’s the speed of AMR that makes our global dependence on antibiotics dangerous, outpacing our capacity to respond by creating new drugs – a complex, costly and time-consuming business that Prof Lithgow says is unsustainable.
If we want antibiotics to retain their power to safeguard surgery and fight infections that, less than a century ago, were death sentences, he says that we need to work to restore the balance in favour of drug-sensitive microbes.
“In any environment – your garden, hospital, school, wherever – you want as many bacteria as possible to be sensitive to the drugs currently on the market,” says Prof Lithgow.
“We can’t afford to increase the proportion of bacteria around you that are drug resistant. When we do that, you’re more likely to be infected by a drug-resistant form that can’t be treated, rather than a drug-sensitive form that can be treated.”
Human characteristics are passed down in a process called vertical gene transfer. But bacteria can do what neither humans, animals nor most plants can, gathering genes sideways from their environment in a process called horizontal (lateral) gene transfer.
Microbes splice these extra genes into their chromosomes, so genes from drug-resistant bacteria will transfer that characteristic to the new colony, making it antimicrobial drug-resistant.
“Some bacteria are absolute experts at doing this, others less so. But many that end up antimicrobial resistant are very good at lateral gene transfer, which is how AMR can spread rapidly,” says Prof Lithgow.
Bacteria can also create physical barricades, a defence he says is used by both drug-sensitive and drug-resistant microbes. Some colonies develop a mucus-like barrier of carbohydrate, while others form biofilms; just as a raincoat keeps your clothes dry, the biofilm structure is able to keep antimicrobial attackers at bay.
“The bacteria inside the biofilm are effectively protected by their neighbours and can persist even in the presence of high doses of drugs,” explains Prof Lithgow.
In most cases, antibiotics target the engine room of a bacterium, where its DNA replication and other essential functions happen. To do this, the influx of antibiotic entry into bacteria uses channels that also carry the sugars and amino acids that support life.
Some bacteria adapt to block those ‘influx’ channels; their nutrients are reduced so they’re less ‘healthy’, but they create the strong resistance we think of as superbugs.
Efflux is a process that’s recognised as one of the most important causes of intrinsic antibiotic resistance in bacteria. Efflux pumps eject waste and unwanted materials, including the antibiotics that threaten their survival.
Humans are fighting back
Research teams worldwide are working on next-gen antibiotics that are essential but are neither a fast, nor sustainable, solution to AMR.
The Centre to Impact AMR’s researchers are exploring sustainable solutions to the problem of AMR, including using bacterium-killing viruses called phages, as well as ways to reverse the adaptation process that creates resistance.
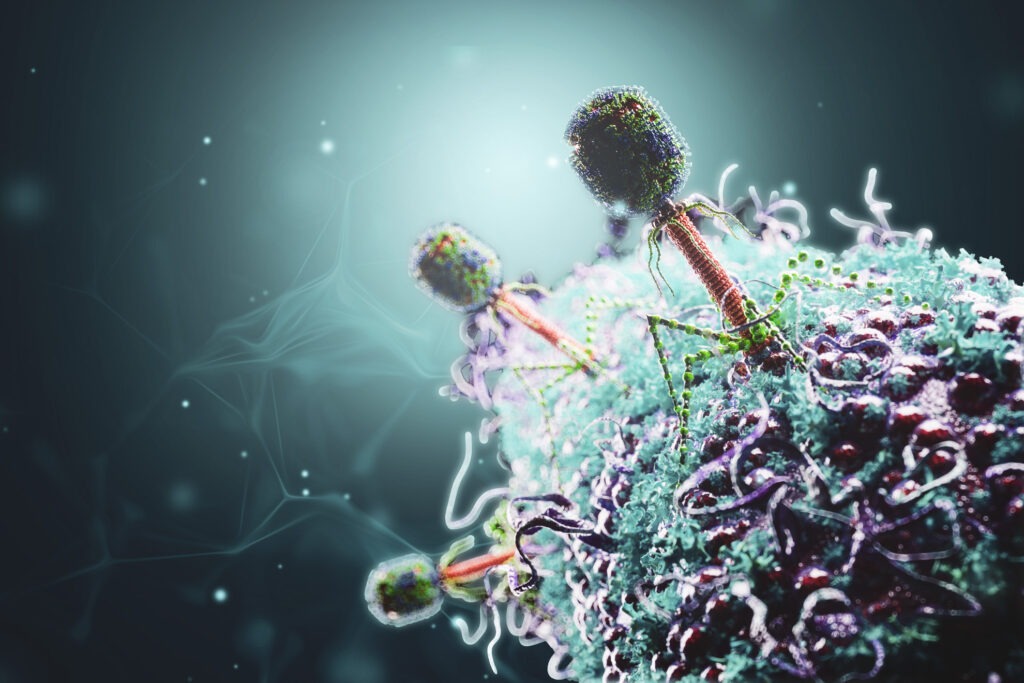
“The idea that when a bacterium becomes resistant to one drug, you get another drug, and then when they become resistant to that you get another drug is unsustainable,” Prof Lithgow says.
One of the most powerful weapons in the battle for microbial balance is AMR stewardship, a program to reduce inappropriate use of antibiotics.
This stewardship in hospitals remain a high priority but, conversely, many of the domestic anti-microbial household cleaners themselves contribute to escalating resistance, as can the use of antimicrobials in animal care and agriculture.
AMR stewardship in action for animals
Dr Stephen Page is founder and director of consultancy Advanced Veterinary Therapeutics. An adviser on the appropriate use of veterinary medicines, including antibiotics, he defines antimicrobial stewardship (AMS) in a veterinary context as “actively looking for ways not to need antibiotics”. Antibiotics that are needed are used according to the 5R AMS model:
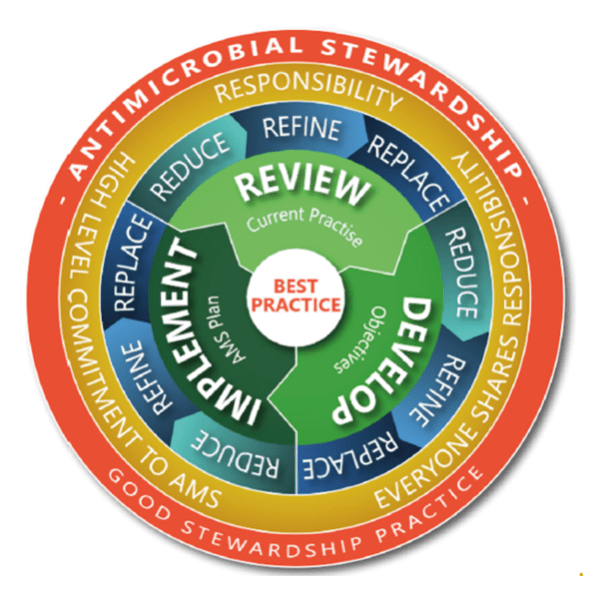
“That’s different to the human approach to stewardship, where they say, ‘We want to use them well’. Of course, [vets] want to use them well too, but only when needed, and then under AMS principles.”
Dr Page says that concerted AMS in agriculture began in the 1990s, with regulatory restrictions limiting antibiotic classes available for use, and prescribing guidelines leading to refined use in support animal health and welfare.
“For instance, the fluoroquinolones are broad-spectrum antibiotics considered critically important for human use,” says Dr Page. “They’re widely used in livestock in other countries, but they’ve never been used in livestock in Australia, though available for use in companion animals.
“
Many animals have little, if any, exposure to antibiotics.
Dr Stephen Page
“‘Healthy animals make safe food’ has been a mantra for decades. In fact, in many operations, if animals get sick or need antibiotics, it is considered a management failure, to be investigated. Many animals have little, if any, exposure to antibiotics,” he says.
“The agriculture industry is not saying, ‘We desperately need a new antibiotic’. Antibiotics are an important and valuable resource, used as little as possible but as much as necessary.”
Michelle Fincke is a Melbourne-based freelance journalist and editor. She has spent the last two years working with case studies and writing in the public health space.